Halogen cooktops (infrared hobs)
by Chris Woodford. Last updated: June 7, 2024.
It takes a mere three minutes to boil an egg—but, ironically, it can take two or three times longer than that for your stove to get hot enough for cooking in the first place. That's the trouble with electric cookers: unlike gas, which makes a flame as soon as you turn it on, electric cooktops (hobs) take quite a bit of time to get hot. Halogen cooktops give you the clean convenience of electric cooking with all the speed and power of gas. They make heat with a bright burst of red light—but how exactly do they work? How can you cook something with nothing but light?
Photo: An infrared halogen cooktop in action. You can see a bright red light, but most of the energy this halogen "ring" is pumping out is actually heat (infrared radiation). There are four tungsten halogen tubes in a single "ring" like this, running in parallel from one side to the other. (You can just see one at the top, currently unlit, running from top left to top right.) The thin white bar you can see running from top right to bottom left is a metallic thermostat that switches the tubes on and off to keep the "ring" at a steady cooking temperature.
Sponsored links
Contents
Why cook things anyway?
Have you ever stopped to think about the science of cooking? Probably not—but let's think about it now. We cook food to kill harmful bacteria that may be lurking inside it, so the food becomes safe for us to eat. Often food tastes much better cooked too; a light and fluffy sponge cake tastes much more scrumptious than a bowl full of eggs, butter, and flour! Pretty obviously, cooked food is usually hot, while uncooked food is cold. The essence of cooking is to heat food over a period of time that's long enough to kill the bacteria and transform the food's structure or appearance. In short, cooking is all about moving heat from the cooker into the food—but how do red lights move heat?
How does heat move?
If you know anything at all about heat, you'll probably know that it doesn't like to stay in one place. Hot things tend to pass their heat onto other things nearby—and they do this in one of three different ways called conduction, convection, and radiation.
If you touch a car that's been standing out in the sun on a summer's day, you'll feel the heat instantly. The heat from the hot metal flows instantly into your hand by a process called conduction. You may have heard the word conduction linked with electricity and heat conduction is very similar: heat can flow through a material much like electricity can. If you push a metal poker into a red-hot fire, heat will flow into the poker from the fire by conduction. And it will keep flowing into the poker until it's as hot as the fire around it. Take the poker out of the fire and plunge it into a bucket of water and you'll see a great whoosh of steam. Now heat flows from the poker to the water, also by conduction. The process of conduction transfers heat between two things that are in direct contact.
Not all heat moves by conduction. If you've ever heated a pan of soup on top of your stove, you'll have noticed how it soon starts bubbling. That's because heat is flowing through it by another process called convection. The soup at the bottom of the pan is closest to the hot stove. It warms up and, because hot materials are less dense (effectively lighter) than colder materials, it starts to rise upward. When it gets to the top of the pan, it cools and falls back down. Meanwhile, more soup is starting to rise up from the bottom of the pan and take its place. So there's a constant pattern of warming and cooling that gradually moves heat throughout the pan. This convection process is how heat travels through fluids (liquids and gases) that are near to something hot. The fluids carry the heat systematically away from the heat source a bit like a conveyor belt.
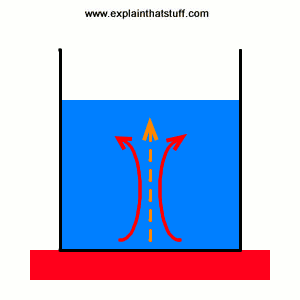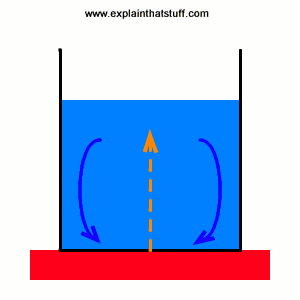
Picture: Convection pumps heat into a saucepan like a beating heart. The pattern of warming, rising soup (red arrows) and falling, cooling soup (blue arrows) works like a conveyor belt that carries heat systematically from the hot stove into the cooler soup (orange arrows). This circulating pattern of liquid is called a convection current.
There's a third way heat can move and it's called radiation. If you've ever sat near a camp fire, you'll know heat beams out from the fire, toward your face, in a direct line. The closer you sit to the fire, the warmer you feel. If anything blocks the direct path between the fire and your face—for example, if someone walks in front of you—you'll notice the difference straight away. Heat radiation is unlike both conduction and convection. It's unlike conduction because you don't have to be touching the heat source (the fire) to feel the radiated heat. And it's unlike convection because there doesn't have to be any liquid or water in between to carry the heat toward you. We can feel radiant heat from the Sun even though most of the vast distance between us and that blazing star is empty space.

Photo: When you heat soup in a pan on a cooktop, you're seeing heat move by conduction, convection, and radiation. Heat conducts from the hot pan into the soup. Heat moves from one part of the soup to another by both conduction and convection. Stand anywhere near the pan and you'll feel heat being given off by radiation.
Infrared radiation is hot light
Why does sunlight feel hot if the Sun is sending out light? Sunlight is actually a mixture of light and heat—of cool light we can see and a kind of "hot light" we can't see called radiation. All hot objects give off radiation in this way. A fire feels hot because a steady stream of infrared radiation beams out from the burning wood and coal and hits our face. Infrared radiation is similar to visible light, but it has a longer wavelength and a lower frequency. In other words, the waves that carry it through the air or space are bigger than light waves and arrive less often. Infrared radiation and visible light (the light we can see) are two kinds of what we call electromagnetic radiation. They're types of energy that travel out as an up-and-down, wave-shaped pattern of electricity and magnetism. X-rays, radio waves, microwaves, and gamma rays are other kinds of electromagnetic radiation. Together, all these things make up what's known as the electromagnetic spectrum.

Artwork: Infrared radiation is a type of light with a slightly longer wavelength and lower frequency than the light we can see (visible light). Artwork courtesy of NASA (follow the link for a bigger version of this image).
What makes one type of electromagnetic radiation different from another? Like light waves, waves of infrared travel at the incredibly fast speed of light: 300,000 km (186,000 miles) per second. It takes just over 8 minutes for the Sun's light and heat to reach Earth, even though it has to travel 149 million kilometers (93 million miles) to get here! There's no real difference between red light and infrared radiation. It's pretty much the same stuff. The difference is simply that our eyes have evolved to see red (and other colors) of light, but they cannot detect the lower frequencies in infrared. Other creatures are built differently. Snakes have special pits in their face that can detect infrared radiation. That means they can hunt at night, even when there's little light about, by detecting the heat that nearby animals give off.
Sponsored links
How to cook with light
The Sun makes Earth warm and light by sending out a mixture of visible light and invisible radiation. Electric light bulbs work the same way. Old-style, incandescent lamps make light when electricity flows through a thin, coiled wire called a filament. The filament gets so hot that it glows white hot and gives off a bright light. This is actually a very inefficient way of making light, because most of the electrical energy the bulb uses is given off as heat and wasted. Energy-efficient lamps work an entirely different way: they use fluorescence to make cool light by crashing atoms together. They use only a fraction of the energy of incandescent lights because they produce very little waste heat.
Cooking with halogen
When you switch on a halogen "ring," the first thing you notice is a dazzling, bright red light as the four tungsten halogen tubes inside start pumping out a mixture of invisible infrared radiation and visible red light toward your food. (Typically, depending on the setting, this gives cooking power between about 150W and 2000W and a temperature of about 700°C or 1300°F—plenty hot enough to cook food. but not so hot that it damages the glass ceramic cooktop.) Unlike with a normal incandescent lamp, where the light is the important thing and the heat is wasted, it's the invisible, radiant heat we're interested in harnessing here. The visible red light is the wasted bit, although it does indicate, very usefully, the power level of the cooking "ring"—the brighter the light, the more infrared heat it's sending, simultaneously, toward your food.
The heat travels out from a halogen "ring" at the speed of light, instantly beaming through the ceramic glass (vitroceramic) cooktop directly above it. The glass is specially designed to transmit about 80 percent of the infrared radiation beaming through it, though it absorbs most wavelengths of visible light (which is why it normally looks dark). If you stand a pot on the glass, it warms up by a mixture of radiation and conduction: heat radiates into the pot from the filaments in the halogen tubes, but it's also transmitted into the pot by conduction from the hot glass just beneath. If you have soup in your cooking pot, it gradually warms up by convection just like with a conventional stove. So, while it's true to say that halogen cooktops work using radiation, they actually cook with a mixture of conduction, convection, and radiation.