
Photochromic lenses
by Chris Woodford. Last updated: June 9, 2023.
Wearing eyeglasses can often be a pain. If it's raining, you're wiping water off the lenses; if it's humid, the lenses mist up; and if it's sunny, you don't know whether to wear your normal glasses or your shades and you may have to keeping switching between the two! Many people who wear eyeglasses have found a solution to the last of these problems by changing over to photochromic lenses—sold under popular brand names such as Transitions® and (some years ago) Reactolite Rapide. They look clear indoors or in poor light, but in sunlight they darken automatically and effectively turn your normal glasses into shades. It's pretty cool technology—but how exactly does it work?
Photo: Definitely darker. I took my photochromic eyeglasses out into the light with the left lens covered, then returned a few minutes later to take this photo. The lenses darken much more given time, but how effectively they work depends on the ambient temperature and how old they are.
Sponsored links
Contents
How normal sunglasses work
Normal sunglasses work by blocking out some of the light in one of two ways. Most of them are really just colored filters: they let through only light of a certain color (the color of the lens) and block out the rest. Since only a fraction of the light gets through, you see a darkened (and colored) picture. The other type of sunglasses use polarization. Light travels in a wave motion—a bit like the waves on the sea. But where ocean waves vibrate only up and down, light waves wriggle in every direction. Polarizing lenses are a bit like slits that let through only light waves vibrating in a single direction. So, just like colored lenses, they let through only a fraction of the light and you see a darkened view of the world (typically grayer, rather than colored).

Photo: Polarizing sunglasses block all light waves except those vibrating in one direction. So if you hold two pairs in front of one another and slowly rotate one of them, you'll see the overlapping lenses gradually turn black, then lighten again.
Photochromic lenses are completely different, because they work by reacting to ultraviolet (UV) light—the light that's just too blue for our eyes to see. Indoors, there is hardly any UV light (ordinary glass windows generally filter it out) so photochromic lenses remain clear; outdoors, where there's quite a bit of UV light coming down from the sun, they darken.
Who invented photochromic lenses?
First let's cut through the jargon. The word "photochromic" comes from two Greek words: "photos" meaning light and "chroma" meaning color. So photochromic simply means something that changes color in response to light.
Photochromic glass has been around since the early 1960s, when it was invented by William H. Armistead and Stanley Donald Stookey of Corning Glass Works (their US patent 3,208,860 describing the idea, titled Phototropic material and article made therefrom, was filed on July 31, 1962). In those days, photochromic glass worked a bit like pieces of old-fashioned, photographic film. Film darkens because it contains silver-based crystals that clump together when light falls on them. Early photochromic lenses contained similar silver crystals and darkened in a similar way: when light hit them, some of the silver crystals changed into microscopic bits of silver.

Photo: Early photochromic lenses contained silver compounds (such as silver chloride) and reacted to light much the same way as old-fashioned photographic film, like this. Unlike film, the reaction was reversible: the lenses soon lightened again.
How can you see through lenses made with opaque silver? As Armistead and Stookey explain in their original patent, only tiny quantities of silver crystals are needed (less than 0.1 percent by volume), and each crystal is less than 0.1 microns (one ten millionth of a meter—or about 100 times thinner than a human hair) in diameter. Unlike photographic film, which darkens permanently, the photochromic lenses could change back again and clear when the light level fell back to normal. In the 1970s, the British Pilkington glass company helped to popularize photochromic lenses by introducing brands called Reactolite and Reactolite Rapide (according to the US Patent and Trademark Office, these two trademarks were applied for in the United States in 1978 and both have since been cancelled).
How modern photochromic lenses work
Modern photochromic lenses tend to be plastic and instead of silver chemicals they contain organic (carbon-based) molecules called naphthopyrans that react to light in a slightly different way: they subtly change their molecular structure when ultraviolet light strikes them. In this altered form, they soak up more ordinary light as it tries to pass by (technically, we say they have a different absorption spectrum), which is what makes the lenses darken. Imagine lots of molecules suddenly darkening inside a clear lens. It's a bit like closing the blinds in front of your window on a sunny day: as the slats turn, they progressively block out more and more light.

Photo: Molecules inside photochromic lenses cut off light like rapidly closing blinds. As the molecules change their structure, they absorb more of the light passing through, and that's what darkens your lenses.
You might think all of this would take quite a bit of time, but photochromic lenses respond remarkably quickly. About half the darkening happens within the first minute and they're cutting out about 80% of sunlight within 15 minutes.
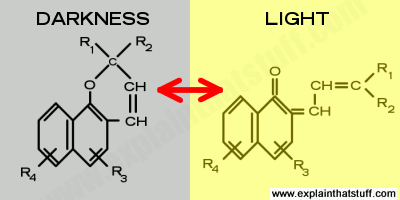
Artwork: When napthopyran molecules are exposed to UV light, their structure changes reversibly. This diagram shows a generic example of such a reaction and is redrawn from Joseph Framingham et al's description in US Patent #3,627,690: Photochromic naphthopyran compounds. You can see that the ultraviolet light powers a restructuring of the molecule on the left, which is cleaved between the carbon and the oxygen atom.
Drawbacks of photochromic glasses
Unfortunately, it takes a little bit longer for photochromic lenses to clear than it does for them to darken in the first place. Generally, they let through about 60% of light again after you've been back indoors for five minutes. However, it can take up to an hour for them to clear completely. You might also be surprised to find that your photochromic lenses darken more or less every time you go outside whether it's sunny or not; that's because they're reacting to ultraviolet light—and there's always plenty of that about even on cloudy days.
A more serious drawback is that the photochromic molecules "react" to temperature as well as light: they darken much more in cold conditions. This means your photochromic sunglasses will give really effective performance in winter (when you probably don't need it) and work somewhat less well in summer (when effective sunglasses are more of a priority). This temperature effect can sometimes be a real problem: the lenses can darken so much that they make driving dangerous in really cold and snowy places, so you're recommended not to wear photochromic lenses for something like driving a snowmobile!

Chart: Temperature matters: You'll probably find your photochromic lenses darken much more in winter than in summer: they get darker, faster in the cold. Chart shows: percentage light transmission after the same time period in hot (top) or cold (bottom) temperatures. Drawn using data from Figure 2.10 of "Chapter 2: Spirooxazines" by Shuichi Maeda in Organic Photochromic and Thermochromic Compounds by John C. Crano and Robert J. Gugliemetti (eds), Kluwer, 2002.
A related problem is that photochromic lenses don't always work effectively in cars, because ordinary glass windscreens naturally screen out most of the ultraviolet light. That means drivers really need a second pair of tinted or polarized sunglasses just for driving in. Although you can get "hybrid" sunglasses that combine photochromism and polarizing in a single lens, the polarization only really works effectively when the photochromism has already darkened the lenses, so they don't work that well indoors (or inside cars) either.
One final difficulty is that photochromic lenses don't last forever. Although they don't actually wear out, after a few years of continuous darkening and lightening, they become noticeably less reactive, particularly indoors. [1] This is less of a problem than it sounds, since many people change their eyeglasses at least this often. (Generally, you should get your eyes tested at least every two years and more often if you're older.) If, like me, you have a stable eye prescription and don't wear your eyeglasses too often, you might find it more of a nuisance: my photochromic lenses seem to have stopped reacting as effectively roughly five years after I bought them.
But all these things aside, photochromic lenses are a brilliant solution for people who need different glasses for different conditions and hate constantly switching between their normal glasses and their shades.
Sponsored links
What are the alternatives?

Photo: Electrochromics (electrically changing lenses) are a promising alternative to photochromics. These are experimental electrochromic (FTPE) sunglasses developed by the Office of Naval Research (ONR). Photo by John F. Williams courtesy of US Navy and DVIDS.
If inventors always think along the same lines, they'll never come up with anything radically new: every idea they have will be a matter of evolution, not revolution. Just as photochromic lenses were a departure from colored filters and polarizing lenses, so there are other options for keeping the sun out of your eyes.
One promising possibility is electrochromic technology, in which small currents of electricity are used to change the orientation of liquid crystals in a thin film, allowing more or less light to get through. LCD sunglasses like this can switch between light and dark much more quickly than traditional photochromics and, because they're manually controlled (at the push of a button), tend to work better indoors (in things like cars) where photochromics struggle to perform in the lack of ultraviolet light. A few years ago, The Office of Naval Research (ONR) developed lenses like this that it called Fast-Tint Protective Eyewear (FTPE), which could be used as protective eyeglasses by Navy SEALs.

